Date: 25th March 2019
There are over 6,000 genetic disorders, many of which have debilitating or life threatening consequences. Diagnosis is usually by genetic testing; medical tests that identify changes in chromosomes, genes or proteins and which can take anything from weeks to months to complete.
With current advancements in genome sequence and structure characterisation within the research setting, the question is posed as to how our findings here might translate into the clinic.
Recent work by the Aran lab, based at the Keck Graduate Institute (US), have used their expertise in biomedical devices to design and test an in-field diagnostic biosensor, termed CRISPR–Chip, giving us a potential glimpse into potential new developments in this field.
CRISPR technology is a popular tool for gene editing within research and is now being translated into therapeutic settings in the clinic. The potential role of CRISPRs within direct diagnostic testing, however, is still relatively unexplored.
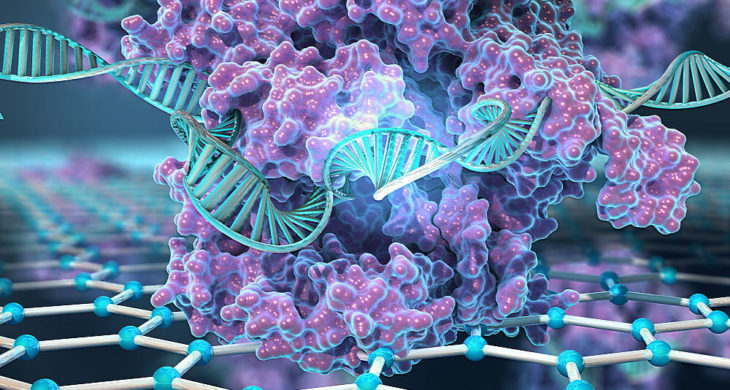
In this report published in Nature Biomedical Engineering Hajian et al. combined CRISPR technology with the ultra-sensitivity of graphene to enable digital detection of a target sequence within an intact genome from human cell lines and clinical samples.
To achieve this a biosensor was constructed by utilising the gene-targeting ability of catalytically deactivated Cas9 protein complexed with a specific targeting single-guide RNA, which was then immobilised on the surface of graphene-based transistors. The idea was that the biosensor would scan the genome for its target DNA sequence and, when bound, the altered conductive properties of the graphene could be subsequently detected with a simple handheld reader.
The so-called CRISPR-Chip, was then successfully used to detect DNA mutations associated with Duchenne Muscular Dystrophy in DNA isolated from HEK293T cell lines and clinical DNA samples.
This work will encourage as many questions around its potential use as much as it delivers innovation. With a testing time of 15 minutes from DNA containing the target sequence to result, without the need for amplification, the system unlocks an interesting promise to harnessing the targeting ability of CRISPR technology for diagnostic testing. Its use as a portable, hand-held device suggests exciting possibilities for greater access to this type of technology within diagnostic settings.
Hajian, R., S. Balderston, T. Tran, T. deBoer, J. Etienne, M. Sandhu, N. A. Wauford, J.-Y. Chung, J. Nokes, M. Athaiya, J. Paredes, R. Peytavi, B. Goldsmith, N. Murthy, I. M. Conboy and K. Aran (2019). “Detection of unamplified target genes via CRISPR–Cas9 immobilized on a graphene field-effect transistor.” Nature Biomedical Engineering.
https://doi.org/10.1038/s41551-019-0371-x
Graphic by Kiana Aran